| Abstract |
Background: HIV increases the risks of malaria in pregnant women, while maternal human immunodeficiency virus (HIV) viral load also facilitates perinatal transmission to neonates. Malaria and HIV coinfection has been shown to exacerbate adverse pregnancy complications. Our study was designed to determine the HIV prevalence of pregnant women at an antenatal clinic in Akure in southwestern Nigeria, investigate the relationship between dual HIV and malaria infection and HIV viral load and CD4+ T cell counts. The study also estimated the risks of adverse pregnancy outcomes in a selected cohort of 74 pregnant women. Materials and Methods: We evaluated the HIV serostatus of 3,225 pregnant women, who attended the antenatal clinic between August 2012 and April 2013. A cohort of 74 pregnant women was selected for the investigation of the relationship between coinfection of HIV and malaria and HIV viral load and CD4+ cell counts. Their HIV status was determined during three trimesters of pregnancy by both HIV-1/2 strips and confirmatory enzyme-linked immunosorbent assay (ELISA) method. Malaria parasitemia was determined by Giemsa-stained thin and thick blood smears. CD4 cell count was by flow cytometry using the CyFlow Counter (Partec, Germany). Viral load estimated by Amplicor HIV-I monitor assay. Results: We found 3.53% prevalence of HIV serostatus among the 3,225 pregnant women who were screened. Forty-four of the 74 subjects were HIV positive and 30 were HIV negative controls. The results show HIV infection among the pregnant women reduced the CD4 cells from a mean of 750 cells/ml for HIV negative women to a mean of 363 cells/ml for HIV seropositive women. Additionally the presence of malaria more than doubled the HIV viral load from a mean of 7,270 ribonucleic acid (RNA) copies/ml for HIV positive women without malaria to 15,148 RNA copies/ml for HIV positive women with malaria. Conclusion: In this study, HIV infection significantly increased risk of acquiring malaria in pregnant women (odds ratio (OR) = 2.27). Dual HIV/malaria infections exacerbated adverse pregnancy outcomes
Keywords: CD4, HIV and malaria coinfection, viral load prevention of mother-to-child transmission
| How to cite this article: Ako-Nai AK, Ebhodaghe BI, Osho PO, Adejuyigbe EA, Adeyemi FM, Ikuomola AA, Kassim OO. The epidemiology of HIV seropositive malaria infected pregnant women in Akure Metropolis, Southwestern Nigeria. Ann Trop Med Public Health 2013;6:519-25 |
| How to cite this URL: Ako-Nai AK, Ebhodaghe BI, Osho PO, Adejuyigbe EA, Adeyemi FM, Ikuomola AA, Kassim OO. The epidemiology of HIV seropositive malaria infected pregnant women in Akure Metropolis, Southwestern Nigeria. Ann Trop Med Public Health [serial online] 2013 [cited 2020 Aug 14];6:519-25. Available from: https://www.atmph.org/text.asp?2013/6/5/519/133703 |
| Introduction |
The human immunodeficiency virus (HIV) and malaria infections have overlapping distributions in sub-Saharan Africa and southeast Asia. [1] They also represent the most prevalent infections in women of child-bearing age. Studies have shown that HIV infection increases the risks of malaria complications in pregnant women, particularly to the developing fetus. These complications may include miscarriages, stillbirths, premature delivery, and postpartum loss of babies. [2],[3],[4],[5],[6] Bakas et al., [3] and Ryder and Temmerman [4] reported that a major percentage of spontaneous abortions and stillbirths in pregnant women were a result of HIV infections. Van Eijk et al., [6] also reported that HIV increased the risk of malaria in women of all gravidities in Kisumu, Kenya. Ticconi et al., [7] reported that HIV infected women were more likely to develop malaria attacks during pregnancy than seronegative women. They also reported that dual malaria and HIV infections increased the risks of stillbirth; preterm delivery; low birth weight; fetal growth restriction and maternal, perinatal, and early infant deaths. Other studies in southeast Asia and central Africa have reported similar findings. [8],[9],[10] In a separate study, Grimwade et al., [11] investigated the interaction of HIV and malaria infections during an outbreak of malaria in KwaZulu, Natal in South Africa. They found that HIV infection increased by two-fold the risk of severe malaria in adults, along with a six- to eight-fold increase in the risk of death. In a study on the impact of HIV infection on the severity of imported malaria in France, Mouala et al., [12] found that malaria episodes were significantly more severe in individuals with CD4 cell counts of <350/ml. Similar findings of severe malaria episodes were reported by Chalwe et al., [13] in a study of individuals with less than 350 ml of CD4 cells in Zambia. Otieno et al., [14] reported an increased level of severe anemia in HIV positive infants compared to those who were HIV negative. In a study on the effect of malaria on HIV ribonucleic acid (RNA) plasma concentration, Hoffman et al., [15] reported that HIV viral burden was higher in individuals with malaria than in controls without malaria. Brahmbhatt et al., [16] examined the association of placental malaria and mother-to-child transmission (MTCT) of HIV in a prospective community-randomized trial in Rakai District, Uganda. In the 746 HIV-positive mother-infant pairs, the MTCT rate was 20.4%. Placental malaria was more common in HIV-positive than HIV-negative women. While malaria and HIV are endemic and have been extensively investigated in Nigeria, no study has been undertaken to evaluate the adverse effects of dual infections in pregnancy. Our study was therefore designed to determine the HIV prevalence in a population of pregnant women at an antenatal clinic in Akure in southwestern Nigeria, investigate the relationship between dual HIV and malaria infection and HIV viral load and CD4+ T cell counts. The study estimated the risks of adverse pregnancy outcomes in a selected cohort of 74 pregnant women.
| Materials and Methods |
Study center
The study was carried out between August 2012 and June 2013 at the Ondo State Specialist Hospital in Akure the capital city with an estimated population of 387,087. A total of 3,225 pregnant women were screened with informed consent for their HIV serostatus as part of their admission to the antenatal program of the hospital. They were also informed that one of the goals of the screening program was to determine the prevalence of HIV infection among the pregnant population enrolled at the clinic and to provide antiviral drugs to those who were HIV seropositive for their immediate clinical management. A cohort of 74 pregnant women was selected from the antenatal clinic population for our study on the interaction of dual HIV/malaria infections on malaria parasitemia and severity and on CD4+ T cell counts and HIV viral load. Approval, ethical clearance, and consent were obtained from the hospital management and ethical board. As part of the informed consent, the goal of the study was carefully explained to each participant in their native language and dialect. Results of their HIV serostatus were provided to the participants by counseling officers, with additional support from social work personnel and nurses of the HIV clinic. Relevant information on age, marital status, and privigrade for each participant was obtained from verbal interview, questionnaire responses, and their case files.
Criteria for study inclusion
Of the cohort of 74 pregnant women selected for the study, 44 were HIV seropositive and 30 were HIV seronegative. Registration at the antennal clinic of the hospital as well as the determination of HIV serostatus at the HIV screening center was required for enrolment in the study. Additional requirements for HIV seropositive subjects included participation in the highly active antiretroviral therapy (HAART) during the course of their pregnancy.
HIV and malaria screening
Blood was collected by venepuncture from each participant. A measured small aliquot of the blood was applied onto the HIV-1/2 strip (Determine Test, Alere, UK) for preliminary HIV determination. Confirmatory test for HIV infection was performed by the Abbott DETERMINE ELISA method (Abbott Labs, Chicago, IL, USA). Thin and thick blood smears for malaria parasitemia were prepared, Giemsa stained and examined under the microscope with oil immersion. Plasmodium falciparum was the malaria parasite identified in all slides. Malaria parasite density was determined by the number of parasites counted against 200 white blood cells. For standard estimation of parasite density per microliter of blood, the number of parasites counted was multiplied by 40 with the assumption of leukocyte count of 8,000 cells/ml. All subjects who were positive for malaria by microscopy and clinical symptoms were immediately placed on antimalarial chemotherapy that consisted of sulfadoxine-pyrimethamine.
CD4 T cell counts and HIV viral load
A 20 ml aliquot of blood was processed to generate the CD4+ cell count by flow cytometry (CyFlow Counter SL-3, Partec, Germany) within 6 h of blood collection. A 20 ml volume of CD4+ monoclonal antibody conjugated to phycoerythrin was also added to the blood sample. The mixture was incubated for 15 min at room temperature in the dark, after which 800 ml of no-lysis buffer was added and gently vortexed. The blood mixture was pipetted to the appropriate port of the CyFlow counter for CD4+ T cell analysis and enumeration. A multiset software was used to obtain absolute CD4 cell counts. For the determination of the HIV viral load, plasma was separated from whole blood by centrifuging at 3,000 rpm for 5 min at room temperature, aliquoted and stored at -80°C until it was needed for analysis. The HIV-1 RNA concentrations were determined by quantitative nucleic acid sequence-based Amplicor HIV-I monitor assay (Roche version 1.5).
Statistical method
Statistical evaluation of data was carried out using paired t-test and analysis of variance (ANOVA) using Statistical Package for Social Sciences (SPSS) V16.
| Results |
The HIV status of the 3,225 pregnant women who attended the antenatal clinic of the Ondo State Specialist Hospital from August 2012 to April 2013 is presented in [Figure 1]. It shows the number of new enrolees, the number of HIV seropositive women and the number of those placed on HAART for each month of the study period. The results show that 114 (3.53%) of the 3,225 pregnant women were HIV seropositive. All HIV seropositive women were placed on HAART as soon as their serostatus was known. The 3.53% HIV seropositive prevalence obtained in our study was significantly higher than the 2.3% prevalence published by the Ondo State Agency for the Control of acquired immunodeficiency syndrome (AIDS) in 2011.
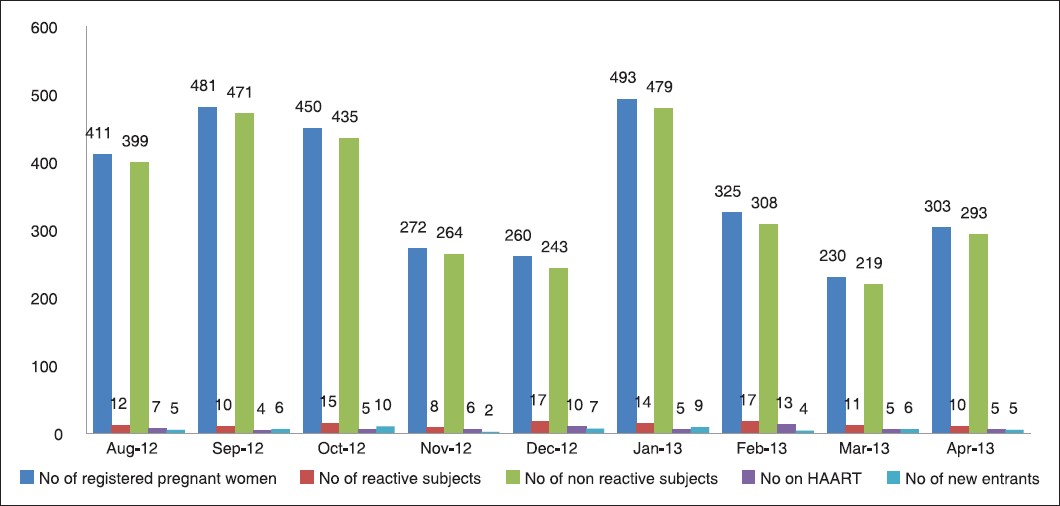 |
Figure 1: Human iunodeficiency virus (HIV) serostatus of pregnant women who attended the antenatal clinics of the Ondo State Specialist Hospital in Akure, HAART = Highly active antiretroviral therapy
Click here to view |
Examination of Giemsa-stained blood smears showed that 34 of the 44 HIV seropositive women had Plasmodium falciparum parasitemia and the remaining 10 women were malaria negative [Table 1]. The blood smears of 18 of the 30 HIV seronegative women revealed falciparum malaria parasitemia, while the remaining 12 women were malaria negative. We classified malaria parasitemia into three groups based on parasite density from the thick blood preparations. High parasitemia included blood smears with over 3,000 parasites/ml of blood, while moderate parasitemia included those with parasite density of 1,000-2,999 parasites/μl. Low parasitemia includes blood smears with less than 1,000 parasites/μl. Episodes of high and moderate parasitemia were recorded for 18 (53%) of the 34 pregnant women with dual malaria and HIV infections, while only eight (44%) of the 18 HIV seronegative but malaria positive pregnant women recorded episodes of high and moderate malaria parasitemia. These episodes collectively included high fever of over 39°C temperature, headaches, myalgia, respiratory difficulty, and convulsion. The calculation for an association showed an odds ratio (OR) of 2.27, indicating that HIV infection increased the risk of malaria and malaria severity in pregnant women. [Table 2] shows profiles of malaria infection in different age groups among HIV seropositive and seronegative pregnant women. In the HIV seropositive group, 86% were between the ages of 20 and 35 years, while the remaining 6% were over 36 years of age. The percentages of moderate to high malaria parasitemia decreased with age, with 91% of 20-25 year group, 83% of 26-30 year group and 60% in the 31-35 year group. The results indicated that the CD4 cell counts in HIV seropositive women declined with the addition of malaria infection, along with a concomitant elevation of plasma viral load. [Table 3] shows the mean CD4+ T cell counts and the mean plasma HIV viral load among the four groups of pregnant women. The 34 women with dual HIV and malaria infections had the lowest mean number of 391 CD4+ cells and the highest mean plasma viral load of 15,148 RNA copies/ml. On the other hand, the women with only HIV infection and without malaria had a mean number of 410 CD4+ cells and a mean viral load of 7,270 RNA copies/ml. The absence of HIV infection increased the CD4+ cell counts to 670-750 cells/ml. Interestingly, the control pregnant women without HIV and malaria had the highest mean CD4+ count of 750 cells/ml, while the control women with malaria had a mean CD4+ count of 670 cells/ml. These results clearly show that malaria infection significantly reduced the CD4+ cell count in HIV seropositive pregnant women and also significantly increased the HIV viral load in HIV seropositive pregnant women. [Table 4] also shows that adverse pregnancy outcomes were most severe in women with dual HIV and malaria infections. Among this group, one woman went into early labor and prematurely delivered a dead baby. Three of the babies born to women in this group died 2-3 days postpartum. Two other pregnancies in this group ended in stillbirth. On the other hand, there were only three adverse pregnancy outcomes among women who were HIV seropositive without malaria with one miscarriage and two postpartum deaths. There were only two premature births among control women who were HIV seronegative. The 44 HIV seropositive women were also examined by their trimester of pregnancy for the relationship between their CD4+ cell counts, their HIV plasma viral load, and pregnancy outcomes. There were four women in their first trimester of pregnancy, 10 in their second trimester and 30 in their third trimester. [Table 4] shows a significant decrease and lowest mean CD4+ cell count of 373 cells/ml as well as a concomitant increase in and the highest mean HIV plasma viral load of 105,783 RNA copies/ml among women in their trimester of pregnancy. There were no adverse pregnancy outcomes among the women in their first trimester, and with only two postpartum deaths among women in their second trimester. Most of the complications occurred among women in their third trimester with one miscarriage, two stillbirths, one preterm delivery with death of fetus, and loss of three babies postpartum.
| Table 1: Frequency and severity of malaria in four different groups of pregnant women
Click here to view |
| Table 2: Profile of malaria parasitemia in different age groups of HIV seropositive and seronegative pregnant women in Akure, Nigeria
Click here to view |
| Table 3: Profile of CD4+ T cells, HIV plasma viral load, and pregnancy complications in four groups of pregnant women in Akure, Nigeria
Click here to view |
| Table 4: CD4+ T cell count, plasma viral load, and pregnancy complications at different trimesters of pregnancy
Click here to view |
| Discussion |
Malaria and HIV represent two of the most important causes of childhood mortality and adult morbidity in southeast Asia and sub-Saharan Africa. Our study examined the relationship between dual HIV/malaria infections and CD4 cells and HIV viral load in 74 pregnant women at an antenatal clinic in Akure, south-western Nigeria. The results show that HIV infection significantly increased the risk of acquiring severe malaria in pregnant women (OR = 2.27). The results also show that HIV infection among the pregnant women reduced the CD4 cells from a mean of 750 cells/ml for the HIV negative women to a mean of 363 cells/ml for the HIV seropositive women [Table 3]. Additionally the presence of malaria more than doubled the HIV viral load from a mean of 7,270 RNA copies/ml for HIV positive women without malaria to 15,148 RNA copies/ml for HIV positive women with malaria. In this study, we defined moderate/high malaria parasitemia as fever with a temperature of ≥38°C, a parasitemia of ≥1,000 parasites/μl, and one of the following: Cardiorespiratory distress, jaundice, hypoglycemia, and impaired consciousness. Eighteen (53%) of the 34 pregnant women with dual malaria and HIV infections experienced episodes of moderate/high malaria parasitemia, compared to 44% of HIV seronegative women with malaria, indicating that HIV moderately increased the severity of malaria in pregnant women (OR = 1.41). Studies have shown that HIV seropositive pregnant women have higher prevalence of asymptomatic malaria than their uninfected counterparts. [6] Our data suggest HIV infection may aggravate the incidence of malaria infection in pregnancy, a view corroborated by previous studies. [17],[18]
One of the goals of our study was to determine the prevalence of HIV among the pregnant women attending the antenatal clinic of the hospital in Akure. We found a prevalence of 3.53% among the antenatal clinic population, which was higher than the 2.3% prevalence for the city of Akure as estimated by the Ondo State Government. The reasons for this difference may be due in part to the female gender composition of the study groups and to the reduced immunity resulting from dual HIV/malaria infections. [19] Overall, four (9.1%) of HIV seropositive pregnant women were screened at the three trimesters of pregnancy, 10 (22.7%) during second and third trimesters, and 30 (68.2%) at the third trimester only. The reason for this lopsided number may be due to the fact that most pregnant women in this part of the world often avoid antenatal clinics except when they suffer from acute or serious ailments. The majority of the women traditionally wait at home until near delivery time before showing up at antenatal clinics, thereby risking pregnancy complications. Our data also show that seven of the 30 women in this category developed complications, underscoring the importance of early detection and monitoring of HIV malaria coinfection. [5],[20],[21],[22] In contrast to HIV seropositive malaria subjects in which 20.5% of the babies died, no death was encountered in both HIV-Mal+ and HIV-Mal- groups. Two of the women in the HIV seronegative groups had premature births but both babies survived. We also compared the frequency of urinary tract infection (UTI) in these groups. All HIV seropositive women had UTI (100%), while 90% of the HIV-Mal+ and HIV-Mal- experienced UTI.
Both innate- and cell-mediated immunity have been reported to be severely affected in HIV infected individuals. Therefore, CD4+ T cell counts and the plasma viral loads of the seropositive subjects were also determined [Table 3]. When the results of CD4+ T cells counts were compared between the HIV seropositive malaria-infected subjects and HIV seropositive malaria uninfected among the first group of women who completed their trimesters of pregnancy, the mean CD4+ T cell count was 408.25 cells/ml and the mean plasma viral load for the group was 876.48 RNA copies/ml compared with 10 of the 44 HIV seropositive women who only participated in second and third trimesters whose mean CD4+ T cell was 444.57 cells/ml mean viral load was 3,310 RNA copies/ml. The third group consisted of 30 women who showed up only at third trimester of their pregnancy. Their mean CD4+ T cell count was 373.32 cells/ml and the mean plasma viral load 105,814.57 RNA copies/ml [Table 1]. Our data shows the mean CD4+T cell level between HIV seropositive malaria infected women in the third trimester of pregnancy (30 women in all) were at high risk of MTCT compared with the control (HIV seropositive malaria uninfected). The (HIV+ MAL+) mean CD4+ T cell count was 363.25 cells/ml compared with 409.86 cells/ml value obtained for HIV+ MAL-. However, their plasma viral load was significantly higher 21,131 RNA copies/ml for HIV+ MAL+ compared to 128.09 RNA copies/ml for HIV+ MAL-. In two separate studies regarding HIV plasma viral load and CD4+ T cell counts, a New York study related HIV plasma load, these investigators recorded a value of 16,000 RNA copies/ml for transmitters and 6,600 for non-transmitters. According to their findings, women with measurable viral load were six times more likely to transmit than those in whom the virus was undetectable after controlling for the CD4 count. [23] However, a European Collaborative Study reported the possibility of increased risk of MTCT where maternal CD4+ T cells counts fell below 700/mm 3 . According to the European Research Group, transmission increased linearly with decreasing CD4+ T cell counts. [24],[25] The French study however showed transmission rates increased with increasing plasma viral load. [25] The French report also showed 12.5% in those with less than 1,000 RNA copies/ml compared with 29% in those with more than 10,000 RNA copies/ml. According to Thea et al., 1997; Cao et al., 1997; [26] and O’Shea et al., 1998, [27] pregnant women with HIV plasma viral load of >50,000 RNA copies/ml at delivery time have been shown to transmit the virus to their newborn. In our study we identified five HIV seropositive malaria infected women with uncommonly high plasma viral loads. The first had plasma viral load of 130,606 RNA copies/ml, delivered prematurely and lost the babe. The second woman plasma viral load was 109,526 RNA copies/ml, lost the baby and also suffered from chronic hypertension. The third woman had pulmonary tuberculosis, was bed ridden and viral load was 90,057 RNA copies/ml. The last two women each had 112,095 and 45,052 RNA copies/ml plasma viral load, respectively. However both women lost their babies. These individuals’ reports underscore the usefulness of high viral load in predicting probable MTCT in a resource limited environment such as existed in this center.
It has been advocated by some investigators that HIV seropositive malaria pregnant women living in areas with stable malaria transmission require effective management of malaria illness through intermittent preventive treatment with sulfodoxine-pyrimethamine or daily cotrimoxazole prophylaxis. [22] Administration of antiretroviral drugs to HIV seropositive pregnant women is intended to reduce their HIV viral loads and to prevent HIV transmission in utero and postpartum. [22] We placed all the 44 HIV seropositive pregnant women at this center on zidovudine (ZDV) and lamivudine (3TC) as an initial mainstay. Additionally we provided nevirapine (NVP) and tenofovir (TDF) according to the need and HIV stage/prognosis of each subject. [28],[29]
Our results suggest HIV and malaria coinfection in HIV seropositive malaria infected pregnant women was more than three-fold higher than in HIV seropositive malaria uninfected subjects. Our data also indicate one in five women among the HIV seropositive malaria infected women is at risk of transmitting the virus to the fetus. The risk of developing complications was greatest in women who reported in their third trimester of pregnancy with about 23.3%. Our studies also provide a vital database and reference point in this region for HIV and malaria coinfection hitherto unavailable to researchers, clinicians, and healthcare providers for effective formulation of intervention strategies for management of HIV and malaria coinfections in pregnant women at the center which hitherto is unavailable. We recognize a significant limitation of our study. Thirty of the HIV seropositive malaria infected subjects only appeared at the clinic during the third trimester of their pregnancy. This group included the majority of the women who developed complications. They also included most of the women whose plasma viral loads were highest, thereby underscoring the fact that late attendance at antenatal clinics contributes to development of serious pregnancy complications.
According to a recent report, Nigeria ranks highest in children acquiring HIV with nearly 60,000 new infections in 2012. [29],[30] To reduce the incidence of HIV and MTCT, morbidity and mortality in general among pregnant women, the state government in the area of study has embarked on provision of limited free antenatal care and medical services to pregnant women in government-run specialist hospitals. It is therefore desirable to reduce the incidence of MTCT of HIV, develop effective strategies to reduce high prevalence rates among majorly illiterate communities where HIV prevalence rates are as high as 30% or more of the population.
| References |
| 1. | Hochman S, Kim K. The impact of HIV and malaria co-infection: What is known and suggested venues for further study. Interdisciplinary perspectives on infectious diseases 2009; 2009:617-954. |
| 2. | Johnstone FD. Pregnancy outcome and pregnancy management in HIV-infected women. In: Johnson MA, Johnstone FD, editors. HIV Infection in women. Edinburgh: Livingstone; 1993. p. 187-98. |
| 3. | Bakas C, Zarou DM, de Caprariis PJ. First-trimester spontaneous abortions and the incidence of human immunodeficiency virus seropositivity. J Reprod Med 1996;41:15-8. |
| 4. | Ryder RW, Temmerman M. The effects of HIV-1 infection during pregnancy and the perinatal period on maternal and child health in Africa. AIDS 1991;5(Suppl 1):S75-85. |
| 5. | Ryder RW, Nsuami M, Nsa W, Kamenga M, Badi N, Utshudi M, et al. Mortality in HIV-1 seropositive women, their spouse and their newly born children during 36 months of follow up in Kinshasa, Zaire. AIDS 1994;8:667-72. |
| 6. | van Eijk VM, Ayisi JG, ter Kuile FO, Misore AO, Otieno JA, Rosen DH, et al. HIV increases the risk of malaria in women of all gravidities in Kisumu, Kenya. AIDS 2003;17:595-603. |
| 7. | Ticconi C, Mapfumo M, Dorrucci M, Naha N, Tarira E, Pietropolli A, et al. Effect of maternal HIV and malaria infection on pregnancy and prinatal outcome in Zimbabwe. J Acquir Immune Defic Synd 2003;34:289-94. |
| 8. | Nosten F, ter Kuile F, Maelankirri L, Decludt B, White NJ. Malaria during pregnancy in an area of unstable endemicity. Trans R Soc Trop Med Hyg 1991;85:424-9. |
| 9. | Taha T. el-T, Gray RH. Malaria and perinatal mortality in central Sudan. Am J Epidemiol 1993;138:563-8. |
| 10. | Verhoeff FH, Brabin BJ, Hart CA, Chimsuku L, Kazembe P, Broadhead RL. Increased prevalence of malaria in HIV infected pregnant women and its implication for malaria control. Trop Med Int Health 1999;4:5-12. |
| 11. | Grimwade K, French N, Mbatha DD, Zungu DD, Dedicoa M, Gilks CF. HIV infection as a cofactor for severe falciparum malaria in adults living in a region of malaria transmission in South Africa. AIDS 2004;18:547-54. |
| 12. | Mouala C, Guiguet M, Houze S, Damond F, Pialoux G, Viget N, et al. FHDH-ANRS CO4 Clinical Epidemiology Group. Impact of HIV infection on severity of imported malaria is restricted to patients with CD4 cell counts <350 cells/µl. AIDS 2009;23:1997-2004. |
| 13. | Chalwe V, Van geertruyden JP, Mukwamataba D, Menten J, Kamalamba J, Mulenga M, et al. Increased risk for severe malaria in HIV-infected adults in Zambia. Emerg Infect Dis 2009;15: 749-55. |
| 14. | Otieno RO, Ouma C, Ongecha JM, Keller CC, Were T, Waindi DM, et al. Increased severe anemia in HIV-1 exposed and HIV-1 positive infants and children during acute malaria. AIDS 2006;20:275-80. |
| 15. | Hoffman IF, Jere CS, Taylor TE, Munthali P, Dyer JR, Wirima JJ, et al. The effect of Plasmodium falciparum malaria on HIV-1 RNA blood plasma concentration. AIDS 1999;13:487-94. |
| 16. | Brahmbhatt H, Kigozi G, wabwire-Mangen F, Serwadda D, Sewankambo N, Lutalo T, et al. The effects of placental malaria on mother-to-child HIV transmission in Rakai, Uganda. AIDS 2003;17:2537-41. |
| 17. | Ayisi JG, van Eijk AM, Newman RD, ter Kuiler FO, Shi YP, Yang C, et al. Maternal malaria and perinatal HIV transmission, Western Kenya. Emerg Infect Dis 2004;10:643-52. |
| 18. | Ayouba JG, Badaut C, Kfutwah A, Cannou C, Juillerat A, Gangnard S, et al. Specific stimulation of HIV-1 replication in human placental trophoblasts by an antigen of Plasmodium falciparum. AIDS 2008;22:785-7. |
| 19. | Colognesi C, Halapi E, Jansson M, Hodara V, Steuer G, Tresoldi E, et al. The role of virologic and immunologic factors in mother-to-child transmission of HIV-1. Am J Reprod Immunol 1997;38:197-200. |
| 20. | Temmerman M. Human immunodeficiency virus and women. J Obstet Gynecol 1994;14(Suppl 2):S70-5. |
| 21. | Miotti PG, Chiphangwi JD, Dallabetta GA. The situation in Africa. Ballieres Clinical Obstet Gynecol 1992;6:165-86. |
| 22. | Thea DM, Steketee RW, Bornshlegal K, Brown T, Orloff S, Matheson PB, et al. The effect of maternal viral load on the risk of perinatal transmission of HIV-1. J Infect Dis 1997;175:707-11. |
| 23. | Newell ML, Dunn DT, Peckham CS, Ades AE, Pardi G, Semprini AE,. Risk factors for mother-to-child transmission of HIV-1. Lancet, 1992;339:1007-1012. |
| 24. | Newell ML, Dunn DT, Peckham CS, Semprini AE, Pardi G. Vertical transmission of HIV-1: Maternal immune status and obstetric factors. European Collaborative Study. AIDS 1996;10:1675-81. |
| 25. | Mayaux MJ, Dussaix E, Ispoet J, Rekacewicz C, Mandelbrot L, Ciraru-Vigneron N, et al. Maternal viral load during pregnancy and mother-to child transmission of human immunodeficiency virus type 1: The French Perinatal Cohort Studies. J Infect Dis 1997;175:172-5. |
| 26. | Cao Y, Krogstad P, Korber BT, Koup RA, Muldoon M, Macken C, et al. Maternal HIV-1 viral load and vertical transmission of infection: The Ariel Project for the prevention of HIV Transmission from mother to infant. Nat Med 1997;3:549-52. |
| 27. | O′Shea S, Newell ML, Dunn DT, Garcia-Rodriguez MC, Bates I, Mullen J, et al. Maternal viral load, CD 4 cell count and vertical transmission of HIV-1. J Med Virol 1998;54:113-7. |
| 28. | Kapiga SH, Bang H, Spiegelman D, Msamanga GI, Coley J, Hunter DJ, et al. Correlates of plasma HIV-1 RNA viral load among HIV-1-seropositive women in Dar es Salaam, Tanzania. J Acquir Immune Defic Syndr 2002;30:316-3. |
| 29. | UNAIDS World AIDS Day report 2012. 20 Avenue appia CH- Geneva, 27 Switzerland. UNAIDS, Core slides: Global summary of the AIDS Epidemic; 2012. |
| 30. | Kelland K. Child HIV Infections cut by half in 7 countries in Africa. Reuters, 2013. http:/m.huffpost.com/us/entry/3496189. London. |
Source of Support: None, Conflict of Interest: None
| Check |
DOI: 10.4103/1755-6783.133703
| Figures |
[Figure 1]
| Tables |
[Table 1], [Table 2], [Table 3], [Table 4]



